Abstract
BACKGROUND The major challenge of solid organ transplantation (SOT) is to prevent graft rejection. The chronic use of pharmacological immunosuppression has been associated with increased rates of infection, cardiovascular, metabolic, endocrine disorders, and cancer. The induction of chimerism by donor hematopoietic stem cell transplantation (HSCT) together with SOT is an active tolerance induction approach. Here, we present a new model for inducing transient mixed chimerism in 2 patients undergoing SOT employing a non-myeloablative conditioning (NMA) regimen and a partially T cell-depleted (TCD) graft consisting of CD34+ hematopoietic progenitor cells and CD45RA− memory lymphocytes.
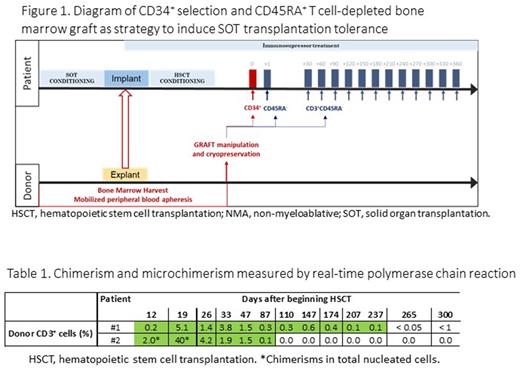
METHODS SOT and HSCT were performed at University Hospital La Paz, Madrid. Bone marrow harvesting was performed on the iliac crest and lumbar and dorsal vertebrae of the deceased donor for patient#1 and mobilized leukoapheresis from sibling donor for patient#2. First we obtained the enriched by CD34+ selection using the CliniMACS system (Miltenyi Biotec) and afterwards the CD45RA− cells were purified. Lineage-specific chimerism analysis was analyzed using quantitative real-time polymerase chain reaction. Immune cell recovery was monitored by multiparametric flow cytometry. We obtained the permission of the Organización Nacional de Trasplantes and the informed consent was obtained from the patient and donor and/or caregivers.
RESULTSPatient 1 A 15-year-old boy was diagnosed with intestinal epithelial dysplasia in 2008. After two previous intestinal and multivisceral acute cellular rejections forced a third MVT. With the imptemption to induce donor tolerance, a T cell-depleted bone marrow graft from the same deceased donor was employed. Bone marrow harvesting was performed obtaining a total volume of 141 ml with 4x109 total nucleated cells. Peripheral blood was obtained after unclasping the thoracic aorta. The stem cell fraction from bone marrow was enriched by CD34+ selection, obtaining 8.75x105/kg CD34+cells/kg. Memory lymphocytes were purified and aliquots containing 1x106/kg CD3+CD45RO+ and 2.1x104/kg CD3+CD45RA+ T cells. Both products were cryopreserved. The conditioning regimen before HSCT included fludarabine and cyclophosphamide. On day 88 after MVT, we infused all the CD34+ cells and 1x106/Kg CD3+CD45RO+ T cells. The CD3+CD45RO+ T cells were also infused periodically on a monthly basis for 1 year. Donor chimerism in CD3+ T cells ranged from 0.05 to 5.1 during the first year after HSCT. We observed a peak of the percentage of regulatory T cells (Tregs). No degree of graft-versus-host disease (GvHD) was observed, and the patient became independent of total parenteral and enteral nutrition. Serial follow-up intestinal biopsies did not show any sign of immune rejection. The patient continued to receive sirolimus to maintain drug concentrations at 6 ng/ml as the only immunosuppression treatment. No signs of rejection were detected after 27 months of follow-up. Patient 2 A 49-year-old woman was diagnosed with chronic kidney failure secondary to rapidly progressive glomerulonephritis. The patient had received 3 previous kidney transplants from deceased donors. A kidney transplant from her sibling was performed in March 2021. Hematopoietic progenitor cells were obtained from mobilized peripheral blood leukapheresis. A NMA conditioning regimen employing total lymphoid irradiation (8 Gy) with antithymocyte globulin (7.5 mg/kg). Two weeks after the kidney transplantation, we infused a graft consisting of 1.27x107/kg CD34+, 4.43x106/kg CD3+CD45RO+, and 5x104/kg CD3+CD45RA+ cells. Controls for hematopoietic chimerism demonstrated the presence of transient mixed hematopoietic chimerism during the first 2 months after SOT of between 0.1% and 40% CD3+ cells. The patient continued on monotherapy with tacrolimus. Immune reconstitution analysis showed a Treg peak. The patient did not have acute rejection, GvHD, viral reactivations or episodes of bacterial infection during the 17 months of follow-up.
CONCLUSIONS We describe for the first time the use of non-myeloablative conditioning and a CD45RA− T cell-depleted graft to induce transitory mixed chimerism using both hematopoietic progenitors and a solid organ coming from the same donor, for tolerance induction purposes. The patients continue to progress favorably 17 months or more after SOT, receiving immunosuppressive monotherapy.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal